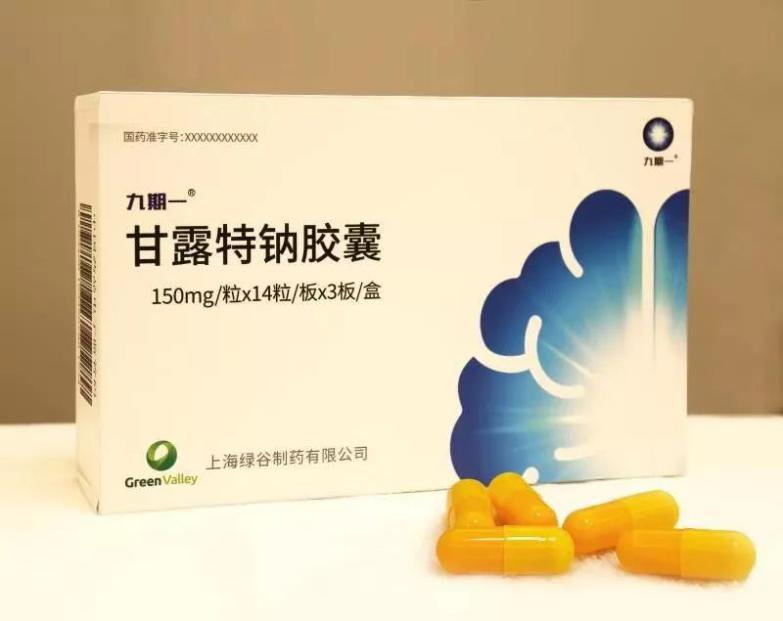
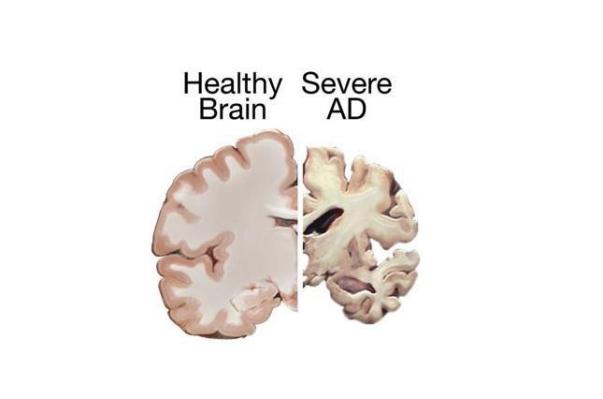
Alzheimer's disease, a central nervous system, is a progressive and progressive neurodegenerative disease of the nervous system. It is characterized by memory disorder, aphasia, loss of use, loss of recognition, visual space skill damage, executive dysfunction and personality and behavior change. Alzheimer's disease is the third major disease that is disabled and fatal in the elderly following cardiovascular and cerebrovascular diseases and malignant tumors. At present, about 10 million people of Alzheimer's disease in China are the largest in the world. With the aging of the population, the number of Chinese patients is expected to reach 40 million by 2050 . The main inventor of the "Nine-phase One" medicine, the research fellow of the Shanghai Institute of Drug Research of the Chinese Academy of Sciences, said that the "Nine-phase One" can obviously improve the cognitive function of mild and moderate Alzheimer's disease, and said: ""The primary efficacy index is very significant and will take effect quickly and steadily around the first four weeks. The drug also has a large feature, which is relatively better for moderate-to-moderate patients. In addition, the drug is very safe."
After the drug was approved, Lu Songtao, chairman of Shanghai Green Valley Pharmaceutical Co., Ltd., said that the company is ready for production and sales and will put the drug on the market this year. "on November 7, we are going to go into production for the first batch, and by December 29, we will spread all the drugs to all channels throughout the country."
(Picture Source:Sogou)
However, Alzheimer's disease is a chronic degenerative disease, which has a long course of disease and is difficult to cure. The Drug Review Center of the State Drug Administration requires that the drug should continue to be studied for long-term safety and effectiveness after the drug is put on the market. Geng Meiyu said that the next step will continue to follow up on clinical data research. "the first thing after the new drug is on the market is clinical reassessment, and second, we need to do real-world research to focus on some of the other chronic diseases in each patient."

On Nov. 2, a new drug for Alzheimer's disease, Phase 9, known as manutte sodium capsule, passed the approval of the State Drug Administration and obtained permission to go on the market. For the vast number of Alzheimer's disease patients and clinicians, this is a huge surprise, because there are about 10 million Alzheimer's disease patients in China, the largest number of patients in the world. However, there are only five drugs used in clinical treatment in the world, and the clinical benefits are not obvious. And "Nine Phase 1", as the first new drug for the treatment of Alzheimer's disease, which is original in China and is the first in the world to target the brain-intestinal axis, will be the majority of Alzheimer's disease. The provision of new treatments also fills the gap that no new drugs have been on the market for 17 years in this area.
Data show that for more than 100 years since Alzheimer's disease was discovered, there are only 5 drugs for clinical treatment worldwide, but the clinical benefit is not obvious. Over the past 20 years, major pharmaceutical companies around the world have invested hundreds of billions of dollars to develop new therapeutic drugs, but more than 320 drugs entering clinical research have failed. Geng Meiyu said that the new drug research and development is so difficult, just in the pathogenesis of Alzheimer's disease is not clear. ``In fact, the course of disease is very long, 20 years before symptoms, Alzheimer's disease has started to occur;In addition, the pathogenesis is very complex, it is a close to aging. The chronic complex disease, such as three high, including unhealthy living diet, depression, including nerve injury, vascular injury, etc., is involved in the development of senile dementia, not only is a disease of the brain itself, it may still be the interaction of the whole body multi-organs. "To really reach the breakthrough of treatment, it must be a strategy of multi-link overall management."
Since the development of the drug, a total of 1199 subjects have participated in the phase 1,3 phase clinical trial study of the "phase 9 one ". Phase 3 clinical trials were carried out by 34 Grade 3A hospitals in China, led by the Mental Health Center of Shanghai Jiaotong University Medical School and Beijing Union Hospital. On the evening of 2 November, the State Drug Administration approved the nine-issue one listing application, which gave clinical medical experts hope. "Professor Geng Meiyu from the brain-gut axis of such a relationship, the whole body of the environment on the impact of brain disease this. Some aspects of the exploration, for us in drug research ideas to provide more innovative value. The most important health problems we encounter today, including neurodegenerative diseases, cardiovascular and cerebrovascular diseases, tumors, metabolic diseases, and so on, may have this problem, so it will have a wide range of enlightening value for our future research. "